News Archives

Another Advocacy Win! Expansion of Coverage for Lymphedema Compression
Following advocacy by APMA and others, effective January 1, 2024, ready-to-wear, gradient compression garments will be covered for the treatment of lymphedema in the absence of an open ulcer.

Public Notice: Call for Third-Party Comments
The University of Texas Rio Grande Valley School of Podiatric Medicine is scheduled for a preaccreditation site visit December 11–14. The preaccreditation decision will take place at the spring 2024 CPME meeting. Third-party comments concerning the University of Texas Rio Grande Valley School of Podiatric Medicine are due by November 15.

CMS Nursing Facility Recoupments
Members who receive such a recoupment for patients who were under a long-term nursing facility stay (Place of Service 32) using Place of Service 31 are encouraged to contact their administrative defense coverage carrier immediately.

Are You Paying Too Much for Health Insurance?
Did the renewal packet from your insurance carrier bring bad news this fall? Find out now whether your practice could save with APMA Health-Care Solutions.

The Call for Speakers is Now Open for The National 2024 in DC
The Call for Speakers for the APMA 2024 Annual Scientific Meeting (The National), August 8–11, in Washington, DC, is now open! Learn more and submit your proposal to be a part of the can’t-miss event for every podiatrist.

Register Now for The 2023 Fall Virtual Coding Seminar
Registration is now open for The 2023 Fall Virtual Coding Seminar, December 2, 10 a.m.–to 3 p.m. EST. APMA’s coding expert, Jeffrey Lehrman, DPM, CPC, will present. All podiatry professionals are welcome to attend and eligible to earn up to 4.0 credit hours.
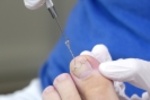
APMA Partners with CAC Representatives and Dermatology to Challenge Proposed Surgical Treatment of Nails Policy
On October 13, APMA supported its Palmetto Jurisdiction Contractor Advisory Committee (CAC) representatives and joined with the American Academy of Dermatology Association (AADA) in submitting comments to Palmetto that raised concerns with proposed Local Coverage Determination (LCD): Surgical Treatment of Nails (DL39258) and proposed Local Coverage Article (LCA): Surgical Treatment of Nails (DA59028).

23rd Annual CAC-PIAC Meeting - Respond to Member Survey by November 1
To ensure that our CAC and PIAC representatives have the fullest picture of the issues facing our membership today, we are asking all APMA members to complete this survey by November 1.

APMA Announces New MOU with Legal and Consulting Firm the Epiphany Group (TEG)
Collaborating with TEG allows APMA members access to services that extend beyond what APMA’s Center for Professional Advocacy (CPA) can provide, such as opportunities for litigation.

Save the Date! 2023 Fall Virtual Coding Seminar, December 2
APMA is excited to announce that there will be a Fall Virtual Coding Seminar. This virtual course will be held on the APMA Online Learning Center on December 2, 10 a.m.–3 p.m. ET.

New Skin Substitute Policies Not Taking Effect
Following advocacy by APMA and many others, Novitas, First Coast, and CGS Part B MACs announced on September 28 that their previously finalized new skin substitute policies “will not become effective on October 1.”

Submit by October 9 - 2022 MIPS Targeted Review Requests
If you participated in the Merit-based Incentive Payment System (MIPS) in 2022, you can review your performance feedback, including your MIPS final score and payment adjustment factor(s), on the Quality Payment Program website.

Call to Action: DEA MATE Act
Please use the APMA’s eAdvocacy website and send our pre-written message about this issue to your legislators to raise their awareness and request support for passage of corrected language in the MATE Act.

New ICD-10-CM Code Set Takes Effect October 1
Providers must use the new code set for services provided on or after October 1. Register now for APMA's December 14 webinar covering podiatry-relevant coding changes. APMA Coding Resource Center (CRC) subscribers can view a full list of podiatry-relevant changes now on the CRC home page.

Coding Refreshers Webinar Series
APMA has created a new webinar series titled Coding Refreshers. This series currently has six coding topics the viewer may already have encountered but now has the opportunity to review.

Skin Substitute Policies Effective Date Delayed
MACs state that the delay to the effective date is due to a large system update.

Falls Prevention Awareness Week: September 18–22
It is Falls Prevention Awareness Week! Join APMA in its efforts to increase awareness surrounding falls, the importance of falls prevention, and the role doctors of podiatric medicine play in this important public health effort.

APMA Participates in National Study to Document Changes in Physician Practice Expense
The AMA is undertaking a new national study, supported by 173 health-care organizations, to collect representative data on physician practice expenses. APMA, through our participation in the RUC, is also participating in the survey.

APMA Comments on CY2024 MPFS, QPP, and OPPS
APMA submitted comments in response to CMS’ Medicare and Medicaid Programs: CY 2024 Payment Policies under the Physician Fee Schedule and Other Changes to Part B Payment, etc., on September 11.

Watch Now: The Value of Young Physician Advocacy
2023 Young Physician Advocate Scholarship winner, Joseph Coppola, DPM, of Medford, MA, encourages you to get involved in advocacy. Watch his video now!